The delivery of medicine, nutrition and gasses via medical device tubing saves lives. Misconnections, however, can have tragic consequences. New designs in enteral feeding connectors are safeguarding patients and improving healthcare.
In any patient setting, adverse events including death can result if medical device tubing is misconnected. A global safety initiative is underway aimed at reducing the risk of patients receiving medicine, fluids or gasses the wrong way. HealthTrust℠ is a supporting organization of the Global Enteral Device Supplier Association (GEDSA) and is among the companies leading the effort to help device manufacturers, clinicians, administrators and supply chain professionals transition to new ISO standard connectors.
Wrong Route Delivery Risks
The FDA reported a case of a child who had both a gastric feeding tube for nutrition and an IV for medicine and hydration. When the patient’s gown was changed, a family member inadvertently attached the IV tubing to the gastric feeding tube. This resulted in medicine being delivered through the feeding tube into the child’s stomach. Fortunately, the event was discovered quickly and there was no harm to the patient.
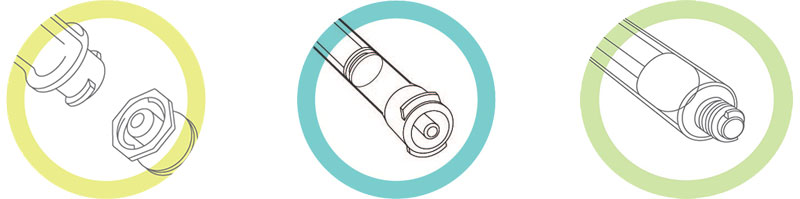
To prevent these types of errors, the International Standards Organization published ISO 80369-3 detailing a safety design for an enteral feeding connector. ENFit® is the first small-bore enteral connector that has come to market. With ENFit, two ends of the connection fit each other but will not connect with any other type of device, making it a connector that will help reduce sentinel events from tubing errors. In California, unless an emergency or urgent situation exists which would impair the ability to render aid, acute care hospitals and other providers are prohibited from using an enteral feeding connector that would fit into a connector other than the type it was intended for.
Mistake Proofing
Poka-yoke is a Japanese term that describes a process of designing products so that they cannot be used incorrectly. Examples are a USB plug that can’t be plugged in backwards, a gas tank filler lid that won’t let you put diesel fuel in your gasoline-powered vehicle, and a micro-wave oven that won’t operate unless the door is closed. Similarly, the ENFit enteral connector is a poka-yoke that promises to reduce the risk of tubing misconnections between unrelated systems through its unique mechanical design.
ENFit devices are ready. Are you?
Organizations have different processes for implementing change, but all require well-informed and prepared teams. The new design standards for medical device tubing, starting with the ENFit connector, are now in place. Please consult the STEPS in the checklists below to help your staff transition to the new enteral connectors. And make it a point to speak with your supplier representatives to learn about availability, timing and indications for use of ENFit administration sets, syringes and feeding tubes.
- Facilities and Institutions
- Home Care Providers
- Nurses and Clinicians
- Patients and Caregivers
- Pharmacies
- Supply Chain Personnel