A new global design standard for medical device tubing connectors will improve patient safety by reducing the incidence of enteral tubing misconnections
A new international design standard for medical device tubing connectors is due to be released this year as part of an initiative aimed at making it difficult, if not impossible, for medical tubing errors to occur.
The Stay Connected Initiative (stayconnected.org) has already begun to yield new connector designs for respiratory systems and driving gases, limb cuff inflation applications, enteral feedings, and neuraxial applications. The initiative is sponsored by the Global Enteral Device Supplier Association (GEDSA), formed in 2013 to help introduce international standards for new enteral connectors.
Newly designed connectors for liquids and gases began coming to market in the past year, with more on the way. Their introduction will affect nearly every level at healthcare facilities, from clinical to supply chain, as inventories of older devices fall and are replaced by the new models.
Tubes or catheters connect patients to medical systems that provide medication, nutrients and fluids. Misconnections—known as Luer misconnections, small-bore misconnections or wrong-route errors—happen when a tube from one system is hooked up to a tube from a different system, such as connecting a feeding system to a tracheostomy tube or an intravenous line.
Errors such as these can result in patient injury and deaths, and likely occur more often than official records indicate, since many mistakes are caught before causing harm and never reported.
Mistakes are often attributed to the universal design of Luer connectors—one of the most commonly used types of small-bore connectors in healthcare. Their simplicity and ease-of-use, however, makes them vulnerable to errors.
Numerous alerts, best-practice recommendations and other educational means are currently used to minimize these errors. Some manufacturers color-code their connectors or offer alternative connectors and delivery systems that are incompatible with Luer connectors. Nevertheless, mistakes continue to happen.
Goals of the New Standards
To further reduce tubing errors, an international group of clinicians, manufacturers and regulators has been working with the International Organization of Standardization and the Association for the Advancement of Medical Instrumentation to develop new ISO standards for connectors. The new standards are intended to ensure:
> greater ability for different manufacturers’ devices to integrate, while making it difficult for unrelated delivery systems to be connected;
> standardized connections across healthcare settings; and
> less likelihood of therapy interruption due to connector incompatibility or unavailability.
Developing international standards will also eliminate the need for individual manufacturers to design and test proprietary designs. This will make it easier to manufacture properly designed components, and free healthcare providers from the need to purchase multiple systems. Color-coding is not included in the 80369 standards. The standards will only address connector shape and size.
The new standards will define the latest connector for enteral applications, called the ENFit. Introduction of this standardized enteral connector will affect currently marketed connectors, including stepped/funnel, ENLock, reverse Luer and other proprietary connectors.
The first of these standards, 80369-1, was announced in January 2011 for connectors used in liquid and gas delivery systems. The new connectors began appearing in late 2014 for liquids and gases.
New standards are in the works for breathing systems and driving gases, enteral, limb cuff inflation, neuraxial and intravascular-hypodermic applications.
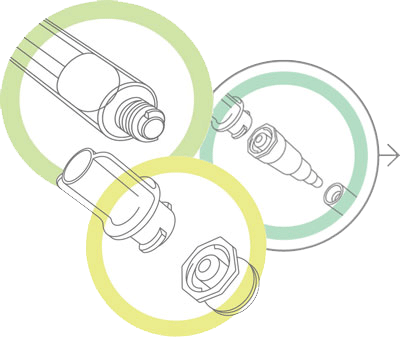 The new designs will be phased in over time to help healthcare facilities better manage inventories of older connectors. (GEDSA has a helpful timeline for the transition here: stayconnected.org/#meet.) During this time, healthcare facilities will need to educate and train personnel to use the redesigned connectors.
The new designs will be phased in over time to help healthcare facilities better manage inventories of older connectors. (GEDSA has a helpful timeline for the transition here: stayconnected.org/#meet.) During this time, healthcare facilities will need to educate and train personnel to use the redesigned connectors.
Supply chain leaders and clinicians can play a significant role in this initiative by staying up-to-date on the new standards’ progress and product availability and passing this intelligence on to the rest of the healthcare facility staff.
The Stay Connected Initiative recommends forming teams to assess existing systems, processes and protocols that may need to change, especially in high-risk areas where the need to convert to new connectors is most urgent.
“This issue is of significant importance to the HealthTrust membership,” says Cathy Crandall, RN, assistant vice president, Clinical Operations, HealthTrust. “Given the reality of and potential for life-threatening consequences, increased awareness and analysis of tubing misconnection errors—including averted errors—can lead to dramatic improvement in patient safety.”
Share Email





