The remarkable race for a vaccine: An update on the COVID-19 vaccine approval and distribution process

As we enter the holiday season, many states are seeing exponential increases in COVID-19 cases. In fact, many models are forecasting significant growth in cases through the end of the year.
Federal, state, and local governments are urging individuals to continue good hand hygiene, avoid touching your face, practice social distancing, wearing a mask appropriately and avoiding large indoor gatherings. This is particularly difficult during the holiday season when indoor family gatherings are a time honored tradition.
Despite the fact that vaccines often take a minimum of six years (or more) of research and testing before reaching the patient, there are multiple promising developments with the COVID-19 vaccine candidates.
Notably, two vaccines, from Pfizer and Moderna, have recently announced impressive results from Phase 3 clinical trials. Both vaccines have been submitted to the FDA for emergency use authorization.
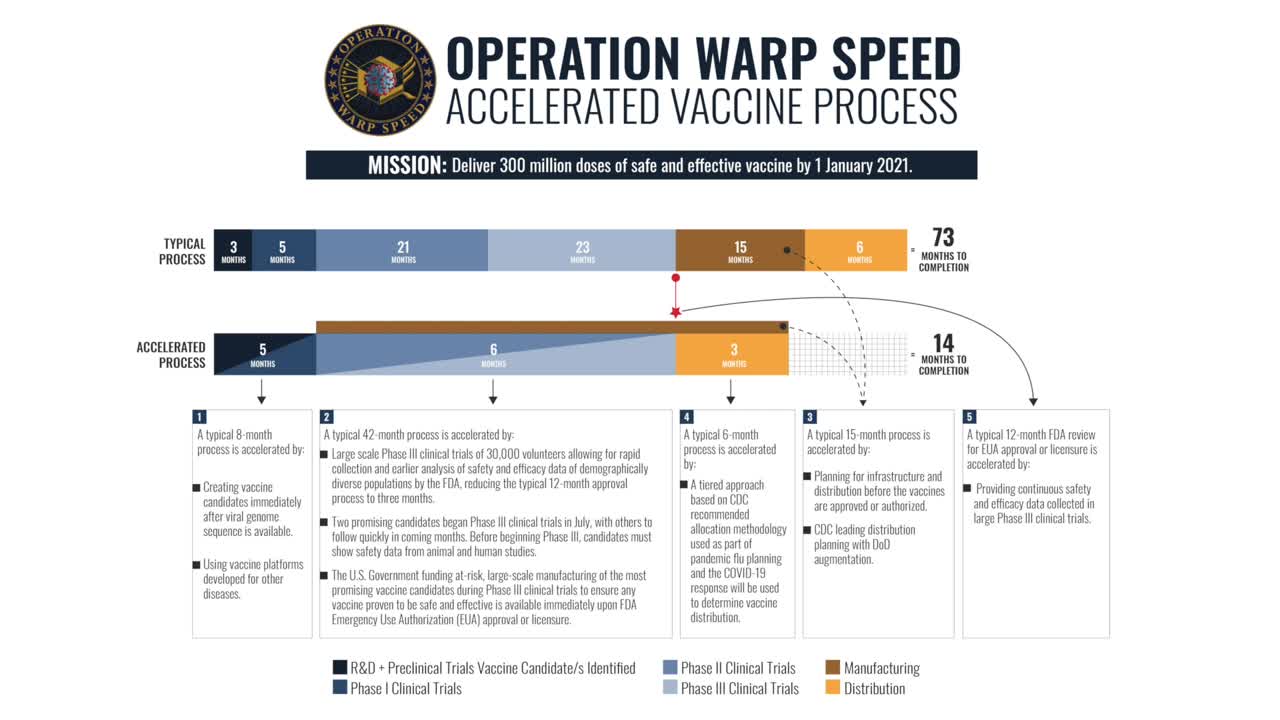
The speed of this vaccine development is remarkable, but also raises safety and efficacy questions. Is it possible to accomplish in 11 months what normally takes 73 months (or more)? Did the accelerated process require clinicians and regulators to cut corners? Will the vaccine, once approved, be safe and effective?

We believe the vaccine will be safe and in this video we answer questions and provide an overview on how the vaccine manufacturers, with the help of the federal government, have been able to move so quickly to the point where we are today.
The most important driver behind the race for a vaccine has been Operation Warp Speed (OWS). With resources from the federal government and the U.S. private sector, OWS has been working since January to accelerate the testing, supply, development and distribution of safe and effective vaccines, therapeutics and diagnostics against COVID-19. Specifically, OWS has set an ambitious goal to deliver 300 million doses of COVID-19 vaccine, with the first doses by January 2021.
In the presentation, we discuss the different types of vaccines that are currently under investigation as well as some of the logistical challenges with vaccine delivery.
Specifically, we look at how OWS has been able to accelerate the vaccine approval process without compromising safety. All COVID-19 vaccines have had to undergo the same rigorous process for approval as any other vaccine – the same clinical trial phases, board review and FDA approvals.

However, the timeline has been compressed through the use of prior vaccine expertise, advancement in bioengineering technology, and government funding. Together, these factors enabled manufacturers to study the vaccines in parallel phases of testing and at the same time begin producing the vaccine with minimal monetary impact. In addition, the defense production act allowed manufacturing to be stood up immediately. As a result, delivery of the vaccine can be initiated as soon as it is approved.
Without cutting corners on safety, OWS has enabled the entire vaccine ecosystem to speedily advance development, testing and manufacturing of these vaccines. To learn more, you can download a PDF of the slides from the talk here.
Share Email Acute Care, COVID-19, Operations, Performance Improvement, Standardization