HealthTrust helps members navigate complex drug approval pathways
It would be an understatement to describe the process of drug approval in the United States as complex. But within the myriad routes to Food and Drug Administration (FDA) approval, two pathways stand out—both of which influence drug adoption, payment, interchangeability and more.

Staying aware & nimble
After successful clinical trials, most new pharmaceuticals are submitted through either a New Drug Application (NDA) or a Biologic License Application (BLA), explains Emily Singleton, PharmD, CAHIMS, Senior Manager of Clinical Information, Pharmacy Services at HealthTrust. Meanwhile, a variety of application subtypes fall under those two primary pathways.
“The FDA’s goal for standard review is to complete the approval process within 10 months, but that can vary a lot,” Singleton notes. “Submission classifications can be added that result in a more rapid time to market.”
HealthTrust members may need to educate providers quickly about a new drug if it’s approved through priority review or accelerated approval, she adds. “HealthTrust may also need to expedite the product review and sourcing process to ensure contract availability at market entrance.”
Managing obstacles
A fundamental understanding of pharmaceutical approval pathways is important no matter what role HealthTrust members play in the healthcare continuum. Why? Invariably, these pathways intersect with aspects that clinicians, staff and hospital leaders all need to manage—including how easily and fully a new drug is adopted, paid for, and substituted or combined with other medications and treatments, Singleton says.
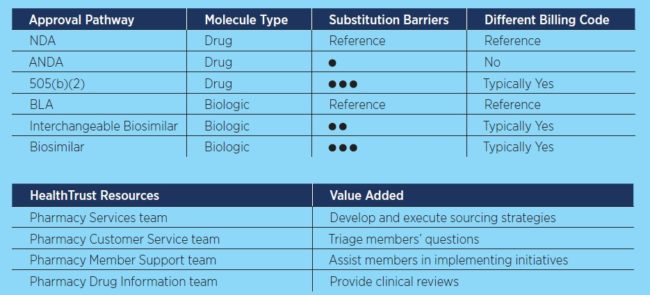
Nuances in the FDA drug application process can translate into how smoothly a healthcare system can integrate a new pharmaceutical into its arsenal. For example, generic drugs, which are submitted under an Abbreviated New Drug Application (ANDA), don’t present many barriers to adoption because of a long history of confident provider use, she explains.
Another drug approval pathway known as 505(b)(2) is becoming more frequently used, Singleton adds. It is a type of NDA for products with some variation from a product already marketed, such as a difference in formulation or administration route. Suppliers may use this pathway as a workaround for remaining patents on the branded product. While providers may treat 505(b)(2) products as generics, the reality is that they have more characteristics of a newly branded drug when it comes to therapeutic equivalency.
Meanwhile, biologics—products derived from living materials that include certain vaccines, blood products and immune system boosters—can be substituted with biosimilars. But this substitution isn’t necessarily straightforward. Without interchangeability status, providers must implement policies for substitution through their medical committees. Although the FDA requires biosimilars to demonstrate that there are no clinically meaningful differences to the reference biologic product, studies that evaluate switching between products are needed for interchangeability status, Singleton says. To date, only two biosimilars have interchangeability status.
Different billing codes are also generated for pharmaceuticals based on the specific FDA approval pathway. This influences how health systems can receive payment for these agents, Singleton says.
“Drugs approved through NDAs and their generics approved under ANDAs have the same billing code, whereas 505(b)(2) products and biosimilars typically all have a different billing code from the reference product,” she explains. “This can make conversion to using a new pharmaceutical more difficult.”
Support when it counts
HealthTrust has developed several resources to help members navigate the drug approval landscape and optimize their own benefits. “Our Pharmacy team incorporates products’ approval pathways into our sourcing strategies. Additionally, our Customer Service team fields members’ questions at any point, while our Pharmacy Member Support team can help members implement sourcing initiatives that may require them to take these drug approval pathways into consideration,” Singleton says. “Our Drug Information team also takes requests for any clinical reviews a member may need.”
HealthTrust can assist members in mastering aspects of drug approval that ultimately can impact clinical outcomes and revenue, Singleton adds.
“Understanding the drug approval pathways can help members better navigate the market and implement clinically appropriate processes, as well as predict future changes in the market,” she notes. “This also helps members understand which differences in pharmaceutical products may be clinical and which may be legal.”
Have a drug question? HealthTrust’s Drug Information team has a list of FAQs posed by the membership. Find a list with answers or ask a new question.
Share Email Biologics, Biosimilars, Drugs, Generics, Q4 22





